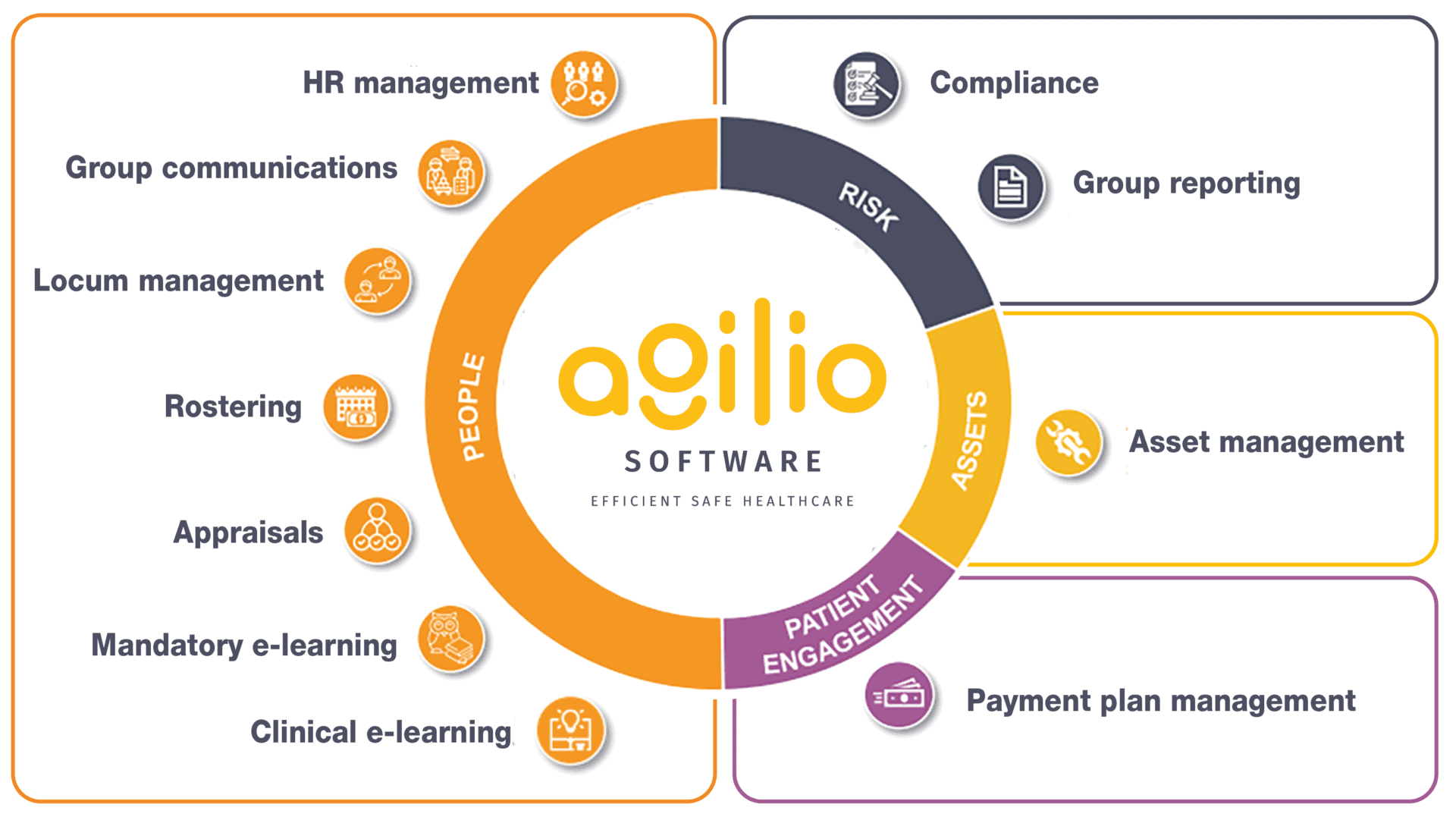
Healthcare Operations are critical to delivering safe and efficient patient care by facilitating communications and coordination, resource management, pay determination, learning and development, quality assurance and compliance, asset management, and data management. By optimising these aspects, alongside your clinical and back-office systems healthcare organisations can enhance patient outcomes, improve patient experiences, and contribute to the overall well-being of individuals and communities.
Agilio Software offers a unified approach to Healthcare Operations. We remove the need to manage the complexities of managing multiple suppliers including the use of a single login to experience a seamless and integrated access to best-of-breed solutions from a single provider. With our comprehensive suite of healthcare operations solutions, your organisation can enjoy a range of benefits that enhance efficiency, quality, and cost-effectiveness.